38 fda and food labels
Pink Sauce goes viral on TikTok: Why has the FDA jumped in? Behind this viral sensation is a chef, who is also a TikToker, and goes by the name 'Chef Pii'. The 29-year-old from Miami had around 800 followers on TikTok. But after she introduced the Pink Sauce, she gained more than 142,000 followers. According to a Los Angeles Times report, Chef Pii sells the sauce at a price of $20 a bottle. Gluten-Free Makeup Brands List - Verywell Health The Food and Drug Administration (FDA) does not issue rules regarding the use of the term "gluten-free" in cosmetics—only food—but will act if a product label is inaccurate or misleading. Report any abuses or concerns to the FDA's Office of Cosmetics and Colors at (240) 402-1130.
FDA Slaps Warning Label On Certain Puberty-Blocking Drugs In July 2022, the U.S. Food and Drug Administration (FDA) placed a warning label on a certain type of puberty blockers known as "'gonadotropin-releasing hormone (GnRH) substances.'"
Fda and food labels
Keto Gummies Reviews: Are Keto Weight Loss Gummies legit? The answer depends on the FDA-approved food label that is attached to your bottle of gummies. Learn what the label says and what it signals when we are talking about expiration dates. It is important to see if the data signals that the food is fatal or is it only listed from a safety point of view. Further, it is also important to take into ... Mexico | Food Safety and Inspection Service Additional information on labels for food products can be obtained from: Direccion General de Regulacion Sanitaria de Alimentos, Secretaria de Salud, Donceles 39, Centro, 06000 Mexico, D. F. Phone: 011-52-5-518-3696. Prepackaged Products The following mandatory information must appear on the labels of prepackaged products: Name of the products. Introductory Courses - Food and Drug Law Institute (FDLI) Introductory Courses. FDLI offers intensive training courses covering the basics of FDA law and regulation. Learn from experienced attorneys and regulatory experts who are highly skilled presenters. The programs are recommended for new, early career, and seasoned staff members working in a range of professional areas, including: regulatory ...
Fda and food labels. Enfamil Formula Recall 2022 & Tampering Issues: What You Need To Know We encourage consumers to check the appearance of the package, the label and the product—even differences that may be minor could be meaningful. If a consumer is uncomfortable using a product they have purchased, they are welcome to call us 1-800-BABY123." Petition to FDA asking for mandatory front-of-package nutrition labels ... In a regulatory petition filed today with the Food and Drug Administration, CSPI is asking the agency to use its authority to establish a simple, standardized, evidence-based, and mandatory front-of-package labeling system for all packaged foods sold in the United States. CSPI is joined by the Association of SNAP Nutrition Education Administrators and the Association of State Public Health ... FDA Infant Formula Update: August 5, 2022 | FDA The FDA is exercising enforcement discretion for the importation of the infant formula products listed above following the review of information provided pertaining to nutritional adequacy and... How GMOs Are Regulated in the United States | FDA U.S. Food and Drug Administration . ... The Standard requires that by 2022, food makers, importers, and certain retailers label foods that are bioengineered or have bioengineered ingredients.
Does Water Expire? FDA Expiration Date, Tap Water The U.S. Food and Drug Administration (FDA) suggests that unopened commercial bottled water is safe indefinitely if the bottles are properly sealed and not broken. The FDA reports that the look, smell, and taste of bottled water may change during long-term storage, but the water is still safe to drink.. While bottled water makers include expiration dates on their labels, these dates are ... FDA Approves Coherus' CIMERLI™ (ranibizumab-eqrn) as the - CIMERLI™ is Coherus' third FDA-approved product and the first of four new product launches planned by the end of 2023 - - First CIMERLI™ product sales expected in October 2022 - AstraZeneca, Daiichi Sankyo win FDA label expansion for Enhertu (NASDAQ ... The U.S. Food and Drug Administration (FDA) on Friday approved the cancer medication Enhertu, developed by AstraZeneca (NASDAQ:AZN) and its Japanese partner Daiichi Sankyo (OTCPK:DSKYF) (OTCPK ... FDA Law Blog TE Codes, FDA explains, are assigned for multisource prescription products based on pharmaceutical equivalence, bioequivalence, and product safety and efficacy profile for the conditions of use specified in the labeling. FDA goes through each of the relevant terms and the therapeutic equivalence requirements.
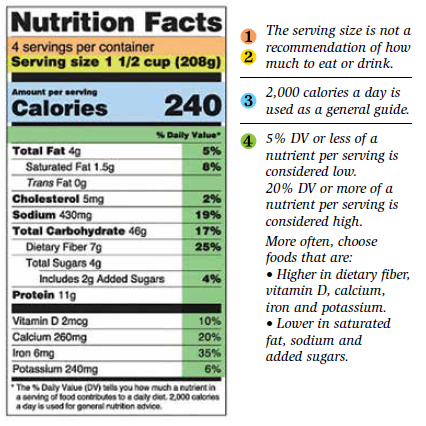
Healthcare Professionals | FDA FDA has updated the Nutrition Facts label on packaged foods and beverages based on current scientific information, new nutrition research, and input from the public. This is the first major update... Food recalls, foodborne outbreaks: What's the difference? A food recall is the removal of contaminated products from the market because of a health risk. However, it is not true that all outbreaks will lead to recalls or that all recalls will lead to foodborne outbreaks. Circumstances can be very specific depending on each situation. A product recall usually follows a foodborne outbreak, but the ... Drugs@FDA: FDA-Approved Drugs - Food and Drug Administration Click on the Drug Name and Application Number to see information about the drug (for example, regulatory history, labeling, reviews by FDA staff). ... U.S. Food and Drug Administration 10903 New Hampshire Avenue Silver Spring, MD 20993 1-888-INFO-FDA (1-888-463-6332) Contact FDA. National Drug Code Directory - Food and Drug Administration U.S. Food and Drug Administration 10903 New Hampshire Avenue Silver Spring, MD 20993 1-888-INFO-FDA (1-888-463-6332) Contact FDA
Royal Crest Dairy Voluntarily Recalls 2% Chocolate Milk Due to ... This Farmer's 2% Reduced Fat Chocolate Milk is bottled in a food safe, single-trip, polyethylene plastic pint container with a black cap. Label stating "Farmer's All Natural 2% Reduced Fat...
European Union | Food Safety and Inspection Service Contact Andrew Yeung at (240) 402-1541 with questions. The FDA laboratory address is as follows: FDA/ Denver Laboratory - Sample Custodian, 6th & Kipling St., DFC, BLDG 20, Entrance W-10, Denver, CO 80225. ... Carton labels of material intended for pet food manufacture must indicate: the phrase "Raw Material Only for the Manufacture of Pet Food"
A discussion of tara and GRAS status | Food Safety News There are three legal pathways for ingredients to be used in food: generally recognized as safe (GRAS) status, a prior sanction, or an FDA food (or color) additive approval. The status of tara...
Import Alert 40-05 - Food and Drug Administration Domestic and foreign manufacturers of infant formula who intend to introduce new infant formula into interstate commerce must first provide CFSAN's Office of Nutritional Products, Labeling and...
Drugs@FDA: FDA-Approved Drugs - Food and Drug Administration This report displays final approvals and tentative approvals of original and supplemental applications for the two weeks beginning on the earliest date listed below. Some approvals may be added to...
FDA Unveils New Easy-To-Read Nutritional Fact Labels for Food Products | Inhabitat - Green ...
Zoetis receives FDA approval for beef implant products Aug 03, 2022. Zoetis has received expanded label approval from the Food and Drug Administration's Center for Veterinary Medicine on three beef implant products. Synovex Choice is the foundation of new reimplant labels that are now available to feedlot operations, which also include Synovex Plus and Synovex One Feedlot.
Formula Recall 2022 & Safety Alerts: Everything You Need To Know List Of Formula Recalls Similac, Alimentum, and EleCare (first Similac recall 2022) Similac PM 60/40 (second Similac recall 2022) Angel formula recall (2022) Able Groupe formula recall (2021) for HiPP, Holle, Kendamil, and Bioland (Lebenswert) Designed by Nature formula recall (2021) SMA Wysoy formula recall - UK (2021)
International Regulations (IRegs) for Animal Product Exports H. Food and Drug Administration (FDA) for bulk gelatin and collagen shipments. In many cases, the Food and Drug Administration (FDA) is the appropriate authority for the certification of shipments of gelatin and collagen. The pertinent FDA office for certification of gelatin and collagen intended for human consumption may be reached at:





Post a Comment for "38 fda and food labels"