41 fda approved drug labels
FDA-approved drug labeling for the study of drug-induced liver injury However, assessing the DILI potential of a drug is a challenge with no existing consensus methods. We proposed a systematic classification scheme using FDA-approved drug labeling to assess the DILI potential of drugs, which yielded a benchmark dataset with 287 drugs representing a wide range of therapeutic categories and daily dosage amounts. How to Get FDA Approval | Registrar Labeling FDA Approved Products. Manufacturers of drugs and devices that do require FDA approval may include the phrase “FDA Approved” on the product’s labeling, as long as the manufacturer has received a letter from FDA confirming its approval. The FDA logo should not be used on a product’s labeling whether the product is approved or not.
Drugs@FDA: FDA-Approved Drugs Action Date Submission Supplement Categories or Approval Type Letters, Reviews, Labels, Patient Package Insert Note Url; 03/23/2021: ORIG-1: Approval Label (PDF)
Fda approved drug labels
DailyMed DailyMed contains labeling for prescription and nonprescription drugs for human and animal use, and for additional products such as medical gases, devices, cosmetics, dietary supplements, and medical foods. The NLM provides DailyMed to the public and does not accept advertisements. Drug Approval and Labeling | Cancer.Net Off-label drug use in cancer treatment is common for many reasons. First, the FDA often approves drugs for treating only a certain type or stage of cancer. The label only reflects past research when the agent received FDA approval. After approval, researchers may find that it is an effective treatment for other types of cancer. PDF Approved v 1 - accessdata.fda.gov FDA-approved test[see Dosage and Administration (2.1)],with no EGFR or ALK genomic ... ALT = alanine aminotransferase, AST = aspartate aminotransferase, DRESS = Drug Rash with Eosinophilia and ... The following clinically significant adverse reactions are described elsewhere in the labeling. Severe and Fatal Immune-Mediated Adverse Reactions ...
Fda approved drug labels. Drugs@FDA: FDA-Approved Drugs For prescription brand-name drugs, Drugs@FDA typically includes the most recent labeling approved by the FDA (for example, Prescribing Information and FDA-approved patient labeling when available),... "Major" Drug Labeling Changes That Require FDA Prior Approval The following quotation delineates what the FDA considers "major" changes to labeling that: (1) require pre-approval, and (2) can't be done via CBE. Any proposed change in the labeling, except changes designated as moderate or minor by regulation or guidance, must be submitted as a prior approval supplement (§314.70 (b) (2) (v) (A)). . . . Off-Label Low-Flow Sevoflurane: Regulatory Red Herring or Liability ... Once a drug is approved for a specific purpose, the drug can be used for any treatment even if the FDA did not approve that treatment. Using the drug for a purpose not indicated on its FDA-approved label is called an "off-label" practice. 5 Off-label use is allowed by law in the context of therapy, but not allowed for research. The ... FDA Drug Labeling Product Requirements, Guidance - PDG FDA's Guidance for Industry entitled "Help-Seeking" and Other Disease Awareness Communications by or on Behalf of Drug and Device Firms (January 2004) describes two types of drug labeling: FDA-approved labeling, and promotional labeling. An example of FDA-approved labeling is the Professional Package Insert (PPI).
Prescription Drug Labeling Resources | FDA FDA's Prescription Drug Labeling Resources website provides over 150 labeling resources for the Prescribing Information, FDA-approved patient labeling, and/or carton and container labeling for... FDALabel - Bioinformatics Tools | FDA DEA Schedule (e.g., CII, CIII, CIV, CV) NDC Number (e.g., 1234-5678, 12345-678, 12345-6789) Include the labeler code and product code separated by a dash (first two NDC segments) Package code (third NDC segment) is not required (it will be ignored if included) SET ID: Labeling alphanumeric code (e.g., 0836c6ac-ee37-5640-2fed-a3185a0b16en ... FDA Label Search The drug labeling on this Web site may not be the labeling on currently distributed products or identical to the labeling that is approved. Most OTC drugs are not reviewed and approved by FDA,... These highlights do not include all the information needed to use VTAMA ... Advise the patient to read the FDA-approved patient labeling (Patient Information). Administration Instructions • Apply VTAMA cream once daily to psoriasis skin lesions only and avoid unaffected areas of skin. • Wash hands after application unless VTAMA cream is for treatment of the hands.
Drugs@FDA: FDA-Approved Drugs Action Date Submission Supplement Categories or Approval Type Letters, Reviews, Labels, Patient Package Insert Note Url; 01/28/2022: ORIG-1: Approval Label (PDF) FDA Label Search-Ingredient Name FDA Label Search. FDA Home - Search by Active Ingredient: (Type in part or all of active ingredient) ... U.S. Food and Drug Administration. 10903 New Hampshire Avenue Silver Spring, MD 20993 Ph. 1-888-INFO-FDA (1-888-463-6332) Contact FDA. For Government; For Press; Combination Products; New Drugs - List of Latest FDA Approvals 2022 - Drugs.com Company: Dermavant Sciences. Date of Approval: May 23, 2022. Treatment for: Plaque Psoriasis. Vtama (tapinarof) is a topical aryl hydrocarbon receptor (AhR) modulating agent indicated for the treatment of plaque psoriasis in adults. FDA Approves Vtama (tapinarof) Cream for the Treatment of Plaque Psoriasis in Adults - May 24, 2022. Drug Labeling Overview - Food and Drug Administration The openFDA drug product labels API returns data from these submissions for both prescription and over-the-counter (OTC) drugs. The labels are broken into sections, such as indications for use...
Drug Labels | FDA Drug Labels This is a partial collection of labeling submitted to the FDA Center for Veterinary Medicine (FDA CVM) by animal drug sponsors for Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and...
Drugs@FDA: FDA-Approved Drugs Action Date Submission Supplement Categories or Approval Type Letters, Reviews, Labels, Patient Package Insert Note Url; 12/21/2020: SUPPL-34: Efficacy-Labeling Change With Clinical Data
FDA Authority Over Cosmetics: How Cosmetics Are Not FDA-Approved Mar 02, 2022 · FDA also may initiate criminal action against a person violating the law. In addition, FDA works closely with U.S. Customs and Border Protection to monitor imports. Under section 801(a) of the FD ...
Table of Pharmacogenomic Biomarkers in Drug Labeling | FDA The table below lists therapeutic products from Drugs@FDA with pharmacogenomic information found in the drug labeling. The labeling for some, but not all, of the products includes specific actions...
Labeling Information | Drug Products | FDA For prescription drug labeling resources (e.g., Prescribing Information, FDA-approved patient labeling, and carton and container labeling), please see the Prescription Drug Labeling Resources web...
Drugs@FDA: FDA-Approved Drugs Action Date Submission Supplement Categories or Approval Type Letters, Reviews, Labels, Patient Package Insert Note Url; 03/04/2022: SUPPL-22: Labeling-Package Insert

Look for these FD&C (Food, Drug & Cosmetic) artificial dyes* on ingredient labels: Note: This is ...
Resources for Information | Approved Drugs | FDA Feb 24, 2020 · Drugs@FDA includes most of the drug products approved since 1939. The majority of patient information, labels, approval letters, reviews, and other information are available for drug products ...
Drugs@FDA: What's in a Drug Product Label? | FDA Information in Drug Product Labels. description of the drug. clinical pharmacology. indications (uses for the drug) contraindications (who should not take the drug) warnings. precautions. adverse ...
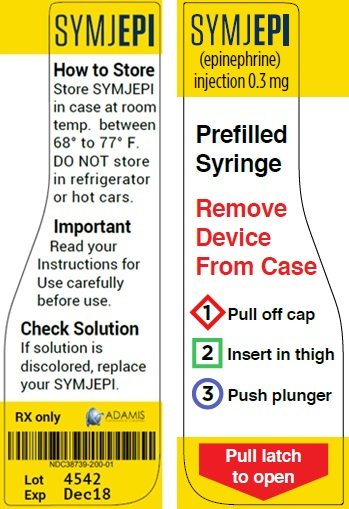
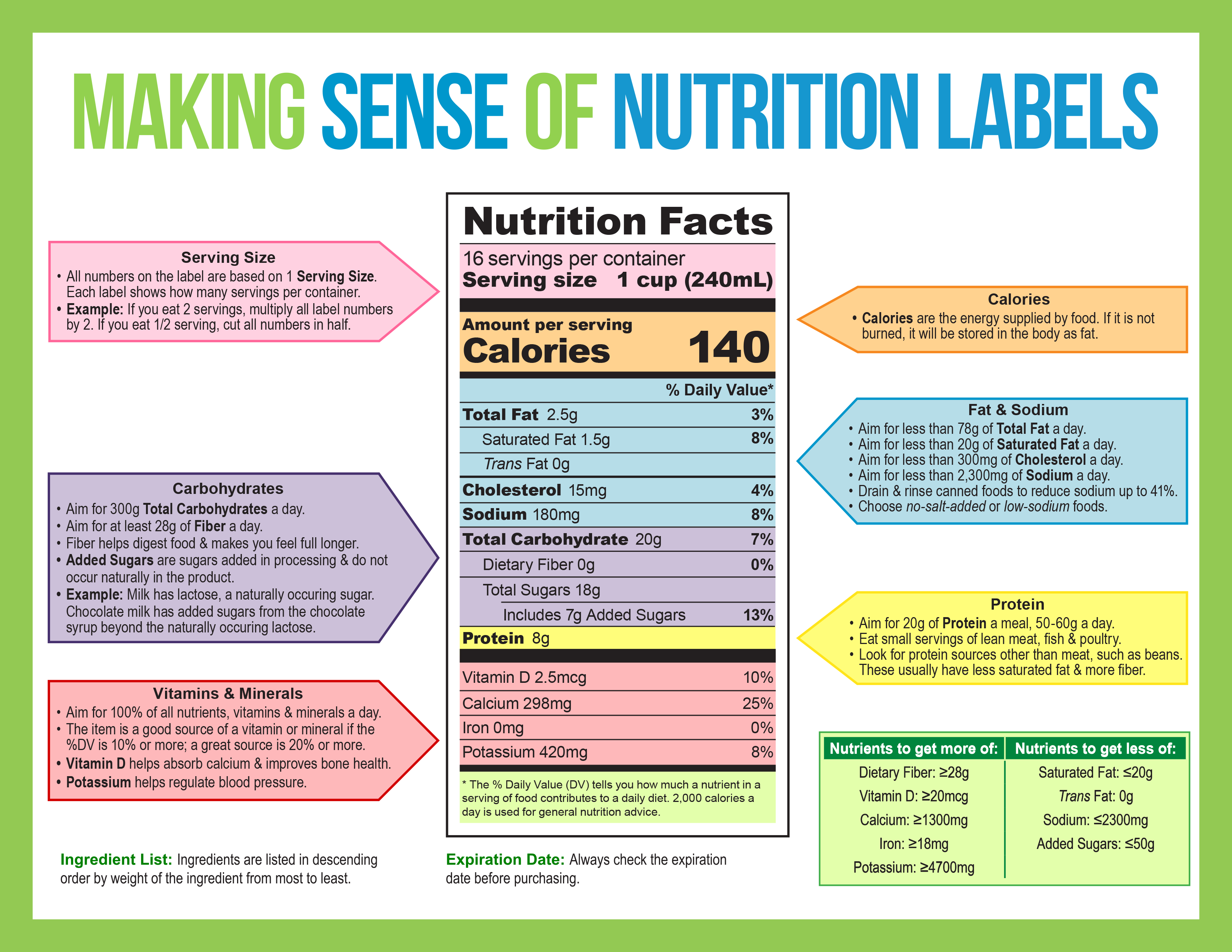

Post a Comment for "41 fda approved drug labels"