39 fda guidance use of symbols on labels
PDF Symbols Commonly Used in Medical Device and Packaging Labeling Caution: Federal law restricts this device to sale by or on the order Use of the "Rx only" symbol as an alternative to the prescription device labeling statement. FDA Guidance Alternative to Certain Prescription Device Labeling Requirements N/A 2. Alarm to indicate an alarm on a control equipment IEC 60601‐1‐8 Table C1, No. 1 3. › documents › 2016/09/21Clinical Trials Registration and Results Information Submission Sep 21, 2016 · Under proposed § 11.44(c), results information submission for applicable clinical trials studying FDA-regulated drugs (including biological products) or devices that were not approved, licensed, or cleared by the FDA for any use before the completion date of the trial may be delayed for up to 2 additional years (i.e., up to 3 years after the ...
› news-events › press-announcementsFDA In Brief: FDA Issues Procedural Notice on Potential Plans ... May 06, 2021 · Today, we are issuing a procedural notice on preliminary consumer research we are planning on the use of symbols on food product labels. The symbol could later be used to convey the nutrient ...

Fda guidance use of symbols on labels
PDF Use of Symbols on Medical Device Labels - FDA Requirements In all such cases, we use EN 980/ISO 15223 symbology on the label, with English-only subtext for each symbol in a small but readable font size. We have been doing this for a number of years now, and have had no objection from US FDA, our NB, our Authorized Representative, authorities in Europe, or end users. adv_webdev24th June 2011 04:45 PM Code of Federal Regulations Title 21 - Food and Drug Administration (c) (1) (i) All words, statements, and other information required by or under authority of the act to appear on the label or labeling for a device shall appear thereon in one or more of the... Summary: Use of Certain Symbols in Labeling | FDA Summary: Use of Certain Symbols in Labeling The proposed rule would provide medical device manufacturers with the option to use standardized international symbols (recognized by the FDA) to...
Fda guidance use of symbols on labels. Code of Federal Regulations Title 21 - Food and Drug Administration The words intended uses or words of similar import in §§ 801.5, 801.119, 801.122, and 1100.5 of this chapter refer to the objective intent of the persons legally responsible for the labeling of an article (or their representatives). The intent may be shown by such persons' expressions, the design or composition of the article, or by the circumstances surrounding the distribution of the article. 21 CFR § 801.15 - Medical devices; prominence of required label ... (4) The meaning or explanatory text for the symbol as provided in the FDA recognition or, if FDA has not recognized the standard or portion of the standard in which the symbol is located or the symbol is not used according to the specifications for use of the symbol set forth in FDA's section 514(c) recognition, the explanatory text as provided ... Professional Labeling - Food and Drug Administration Certain OTC monographs explicitly permit professional labeling. When professional labeling is permitted, it can be provided solely to healthcare professionals. In addition, the product itself must be OTC-monograph compliant and should not have the professional labeling or any representations or claims for the professional use directly on the ... Using Symbols to Convey Information in Medical Device Labeling | FDA The use of stand-alone symbols on a global scale may help promote better understanding through consistent labeling across products distributed in the U.S. and foreign markets. Before this rule, FDA...
› documents › 2022/03/28Federal Register :: Agency Information Collection Activities ... Mar 28, 2022 · (Response 2) FDA has an existing definition for the claim “healthy,” and in the Federal Register of September 28, 2016, we announced our intent to exercise enforcement discretion around some criteria for the claim (see “Use of the Term `Healthy' in the Labeling of Human Food Products: Guidance for Industry,” 81 FR 66527). However, as ... The FDA's Guidance on Labels and Cartons: yet another attack on ... The Guidance goes so far as to almost dictate a 12-point sans serif font size (such as Arial) to improve readability of pharmaceutical labels. In addition, the FDA seeks to define an acceptable ... Guidance for Industry and Food and Drug Administration Staff; Use of ... The guidance document entitled "Use of Symbols on Labels and in Labeling of In Vitro Diagnostic Devices Intended for Professional Use" provides guidance on the use of those recognized symbols. FDA announced the availability of the level 1 draft guidance document in the Federal Register of October 28, 2003 ( 68 FR 61449 ). Use of Symbols in Labeling | FDA Use of Symbols in Labeling The Food and Drug Administration (FDA) issued a final rule, Use of Symbols in Labeling, June 15, 2016, that became effective September 13, 2016. The final rule permits...
› medical-devices › device-labelingUse of Symbols in Labeling: Frequently Asked Questions | FDA The Use of Symbols in Medical Device Labeling Final Rule ("final rule"), including the requirement for a glossary, only applies to symbols that are used to convey information required by or under... › business-guidance › packaging-andPackaging and labelling | Food Standards Agency Mar 24, 2021 · How to display mandatory information on packaging and labels. A minimum font size applies to mandatory information which you must print using a font with a minimum x-height of 1.2mm. If the largest surface area of packaging is less than 80cm squared, you can use a minimum x-height of 0.9mm. Mandatory details must be indicated with words and ... Federal Register :: Use of Symbols in Labeling accordingly, this final rule provides that a stand-alone symbol is allowed to be used in device labeling if: (1) the symbol is established in a standard developed by an sdo; and (2) the standard is recognized by fda under its authority under section 514 (c) of the fd&c act and the symbol is used according to the specifications for use of the … FDA ANNOUNCES GUIDANCE FOR SYMBOL USE ON IVD LABELS - FDAnews FDA ANNOUNCES GUIDANCE FOR SYMBOL USE ON IVD LABELS December 2, 2004 The FDA published a final guidance Nov. 29 outlining recommendations on the use of symbols on labeling for in vitro diagnostic devices (IVDs) to harmonize standards of conveying pertinent and required information about the devices. To View This Article: Login
Device Labeling | FDA Labeling regulations pertaining to medical devices are found in the following Parts of Title 21 of the Code of Federal Regulations (CFR). General Device Labeling - 21 CFR Part 801 Use of Symbols -...
Summary: Use of Symbols in Labeling (Final Rule) | FDA The final rule also specifies that the use of symbols, accompanied by adjacent explanatory text continues to be permitted. FDA is also revising its prescription device labeling regulations to allow...
Code of Federal Regulations Title 21 - Food and Drug Administration CFR - Code of Federal Regulations Title 21. The information on this page is current as of Mar 29, 2022. For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Sec. 801.109 Prescription devices. A device which, because of any potentiality for harmful effect, or the method of its use, or the ...
CFR - Code of Federal Regulations Title 21 For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). § 801.1 - Medical devices; name and place of business of manufacturer, packer or distributor. § 801.3 - Definitions. § 801.4 - Meaning of intended uses. § 801.5 - Medical devices; adequate directions for use.
FDA Requirements for the Use of Symbols on Medical Device Labels Re: Use of Symbols on Medical Device Labels - FDA FDA does require more and more often that all symbols are explained in "english wording". To avoid any problems with your packaging for the rest of the world, one way of proceeding is to put the explanation on a separate label. This is acceptable for FDA.
Use of Certain Symbols in Labeling - Federal Register the food and drug administration (fda) is proposing to revise medical device and biological product labeling regulations to explicitly allow for the inclusion of stand-alone graphical representations of information, or symbols, if the symbol has been established as part of a standard developed by a nationally or internationally recognized …
Professional Labeling (cont.) - Food and Drug Administration It is not possible, in OTC drug product labeling, to provide adequate directions and warnings to enable the layperson to make a reasonable self assessment of these factors [relating to the need for drug therapy and its safety for these purposes]." As described in [21 CFR § 343.80], certain indications, such as cardiovascular-related uses, may ...
FDA Label Search The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.) The drug labeling and other information has been reformatted to make it easier to read but its content has neither been altered nor ...
en.wikipedia.org › wiki › LabelLabel - Wikipedia These labels include information like brand name, class and type designation, and alcohol content. Packaging. Packaging may have labeling attached to or integral with the package. These may carry pricing, barcodes, UPC identification, usage guidance, addresses, advertising, recipes, and so on. They also may be used to help resist or indicate ...
Use of Symbols in Medical Device Labeling- FDA's Final Rule The rule finalizes the FDA's 2013 proposed rule and affects biologics, IVDs, and medical devices regulated by 21 CFR Parts 660, 801, and 809. The revision allows for the inclusion of symbols in labeling without adjacent explanatory text (referred to as "stand-alone symbols"), so long as certain requirements are met.
downloads.regulations.gov › FDA-2013-N-0125-0021Use of Symbols on Labels and in Labeling of In Vitro ... Guidance for Industry and FDA Staff Use of Symbols on Labels and in Labeling of In Vitro Diagnostic Devices Intended for Professional Use Document issued on: November 30, 2004 The draft of this document was issued on October 28, 2003. The information collection provisions in this guidance have been approved under OMB control number 0910-0553.




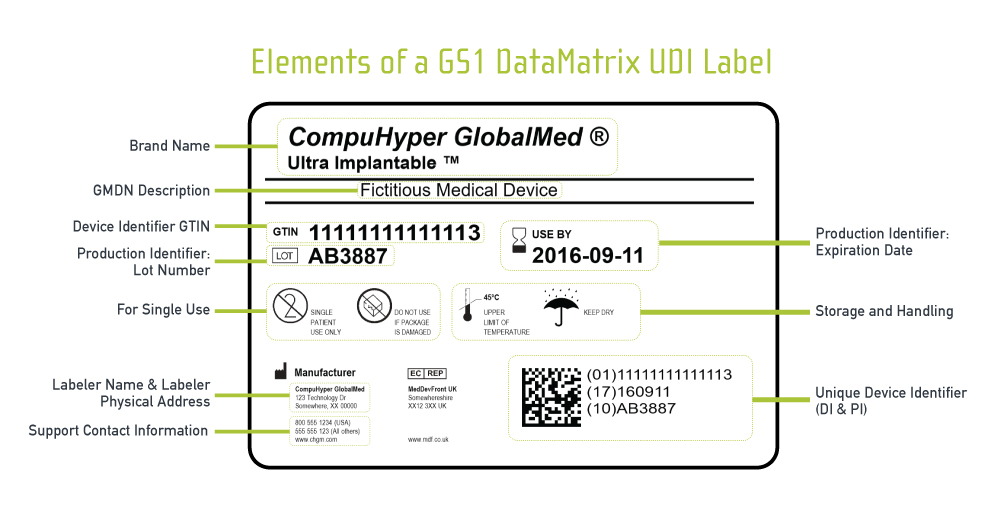

![FDA - Labeling - [PDF Document]](https://reader019.documents.pub/reader019/reader/2020031315/58a1ddb31a28abb6678b698b/r-1.jpg)

Post a Comment for "39 fda guidance use of symbols on labels"